1. Overview
The Hydrothermal Diamond Anvil Cell (HDAC) is a specialized high-pressure–high-temperature (HPHT) optical device designed for in-situ microscopic and spectroscopic observation of fluid systems at geologically relevant P–T conditions.
It enables direct visualization and measurement of phase equilibria, fluid inclusion behavior, mineral dissolution and precipitation, and supercritical CO₂–water–rock reactions up to >1000 °C and >1 GPa.
Originally developed by Prof. I-Ming Chou and collaborators, the HDAC has become a cornerstone instrument in experimental geochemistry, fluid-inclusion thermodynamics, and carbon storage research, allowing scientists to replicate and observe hydrothermal processes as they occur deep within the Earth’s crust.
2. Principle
The HDAC is based on the diamond-anvil cell (DAC) concept, in which two gem-quality diamonds with flat tips (culets) compress a small sample volume contained in a thin metal gasket (typically stainless steel, rhenium, or Inconel).
When pressure is applied mechanically, the sample is sealed between the diamond culets, achieving gigapascal pressures within a chamber volume of only tens of nanoliters.
For hydrothermal applications, the cell is fitted with resistive or laser heating elements and optical access through the diamonds, allowing:
-
Visual observation of phase transitions using a microscope;
-
Raman, fluorescence, or IR spectroscopy for compositional and structural analysis;
-
Precise control of temperature and pressure along a desired P–T path.
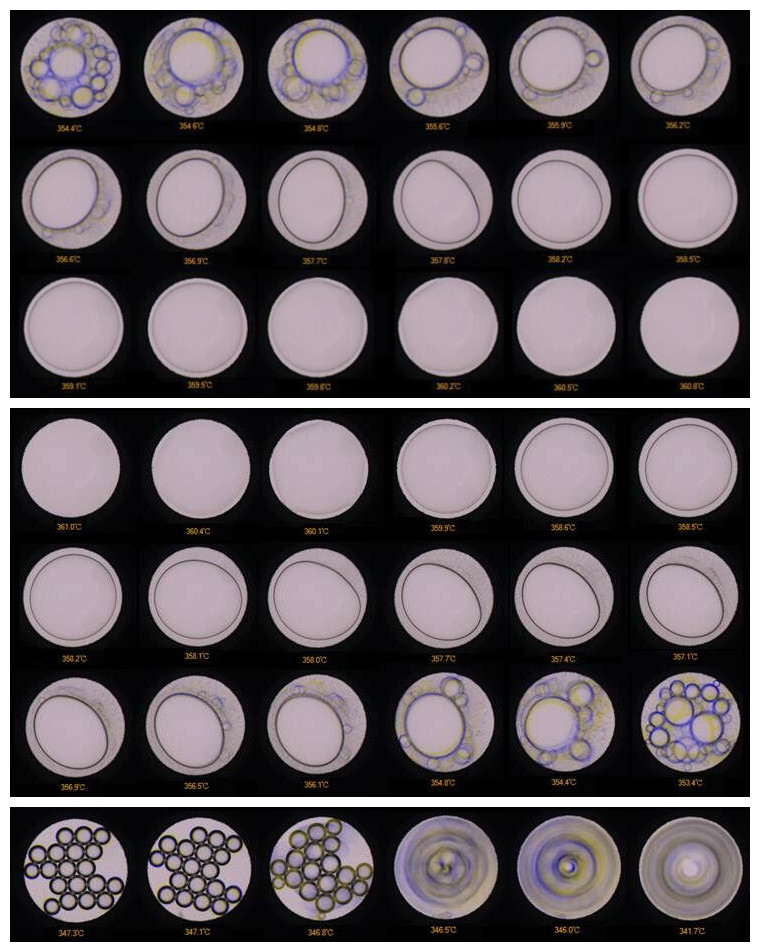
By monitoring optical and spectral changes during heating and compression, researchers determine phase boundaries, critical points, and solubility trends of complex aqueous and volatile systems.
3. System Configuration
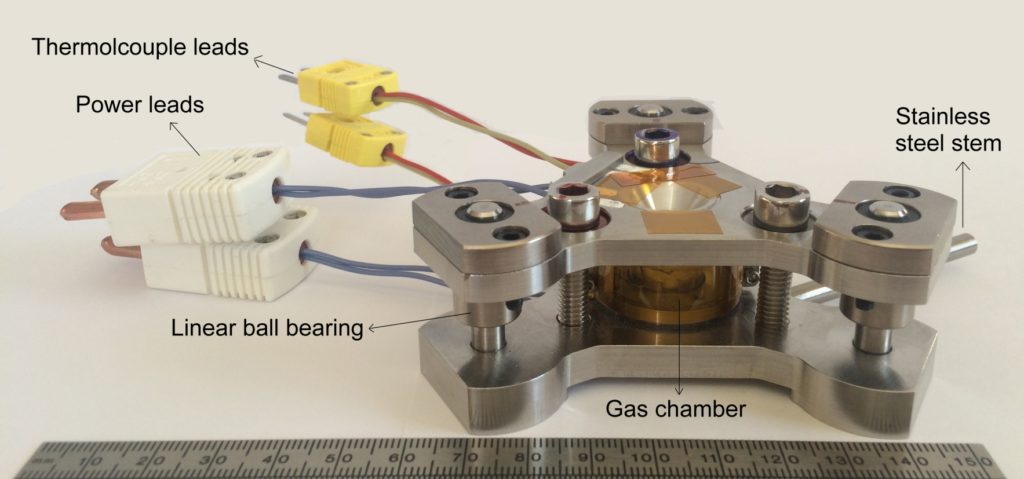
A typical HDAC consists of the following components:
-
Diamond anvils:
Two gem-quality diamonds (0.3–0.5 carat) with 300–600 µm culet size, mounted in tungsten-carbide or steel supports. -
Gasket assembly:
A pre-indented metal foil (100–200 µm thick) with a micro-drilled hole (100–300 µm diameter) forming the sample chamber. -
Sample loading system:
The chamber is filled with a water-rich or CO₂-bearing solution, possibly containing minerals, salts, or hydrocarbons. The sample is sealed automatically by surface tension and gasket confinement. -
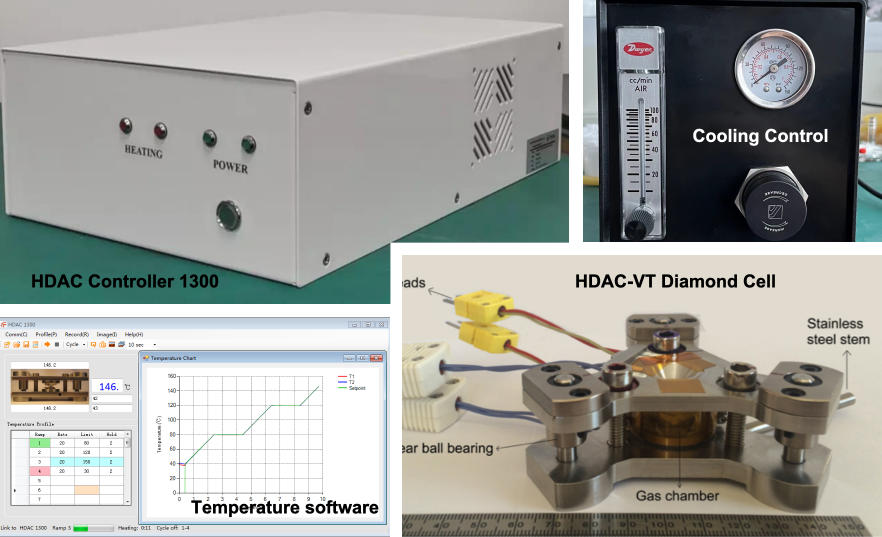
Heating system:
Either external resistive heaters surrounding the anvil seats or focused laser heating to reach 25–1000 °C, depending on the design. -
Pressure generation and calibration:
Pressure is applied mechanically via screws or a pneumatic ram and is calibrated using Raman shifts of reference materials (e.g., quartz, ruby, or SrB₄O₇:Sm²⁺). -
Optical system:
Coupled to a high-magnification microscope (transmitted or reflected light) and optionally to Raman/FT-IR spectrometers through the diamond windows.
4. Experimental Capabilities and Applications
-
Phase Equilibria and Critical Phenomena:
Determine vapor–liquid coexistence, critical points, and immiscibility gaps in H₂O–CO₂–NaCl and other hydrothermal systems. -
CO₂ Solubility and Miscibility Studies:
Visualize dissolution of CO₂ into brines and crude oils, relevant to CCUS and CO₂-EOR. -
Mineral Dissolution and Precipitation:
Observe formation and growth of quartz, carbonates, sulfates, and metal complexes under hydrothermal conditions. -
Fluid Inclusion Re-equilibration:
Recreate and study the homogenization of natural fluid inclusions to determine trapping P–T conditions. -
Spectroscopic Characterization:
Perform Raman and infrared analyses to identify species such as H₂O, CO₂, CH₄, H₂S, and to measure their densities and structural states. -
Thermodynamic Property Determination:
Quantify phase boundaries, solubility, density, and interfacial behavior under confinement or near the critical point.
5. Data and Interpretation
Measurements obtained from HDAC experiments include:
-
Phase boundary curves (e.g., liquid–vapor coexistence, critical temperature/pressure);
-
Homogenization and freezing temperatures of trapped fluids;
-
In-situ Raman/IR spectra revealing molecular interactions, speciation, and density;
-
Visual micrographs of bubble dynamics, mineral growth, and droplet morphology;
-
P–T calibration datasets used to constrain thermodynamic models.
These data are invaluable for developing equations of state, understanding fluid–rock reactions, and improving CO₂ storage and enhanced recovery predictions.
6. Advantages
-
Extremely high pressure and temperature capability (up to 1–2 GPa and 1000 °C).
-
Transparent optical access through diamond anvils for visual and spectroscopic study.
-
Minute sample volume enabling rapid thermal equilibrium and low material consumption.
-
Direct observation of phase transitions and chemical reactions in situ.
-
Compatible with Raman, IR, and fluorescence spectroscopy.
-
Non-destructive and reversible control of P–T cycling for multiple runs on the same sample.
7. Summary
The Hydrothermal Diamond Anvil Cell (HDAC) is a powerful experimental tool that bridges geochemistry, petrology, and reservoir engineering.
By reproducing extreme pressure–temperature conditions while allowing optical and spectroscopic access, it enables real-time visualization of hydrothermal processes that govern CO₂ solubility, mineral formation, and phase equilibria in deep geological environments.
In modern CCUS and EOR research, the HDAC provides unique insights into CO₂–water–rock interactions, fluid inclusion behavior, and supercritical phase properties, contributing directly to the understanding of carbon storage integrity and subsurface fluid dynamics.
Complete HDAC system with heating and cooling control